The Ghost of Methods Past: How Purification Protocols Mask DNA Contamination in mRNA Vaccines and produced a False Negative
12 Substacks of Christmas: Three Fatal Flaws in Achs' "Safe" Vaccine Study
Executive Summary: The “Safe” Vaccine Result Was a Lab Error
Two scientific studies recently analyzed COVID-19 mRNA vaccines to answer a critical safety question: Do they contain too much leftover DNA from the manufacturing process?
Study A (Achs et al.) said NO. They reported DNA levels were very low and safe.
Study B (Speicher et al.) said YES. They reported DNA levels were hundreds of times higher than the limit.
A forensic audit of how these experiments were conducted reveals why the results were so different. The “safe” result from Study A appears to be a false negative caused by aggressive cleaning methods that destroyed the evidence before it could be measured.
Key Findings
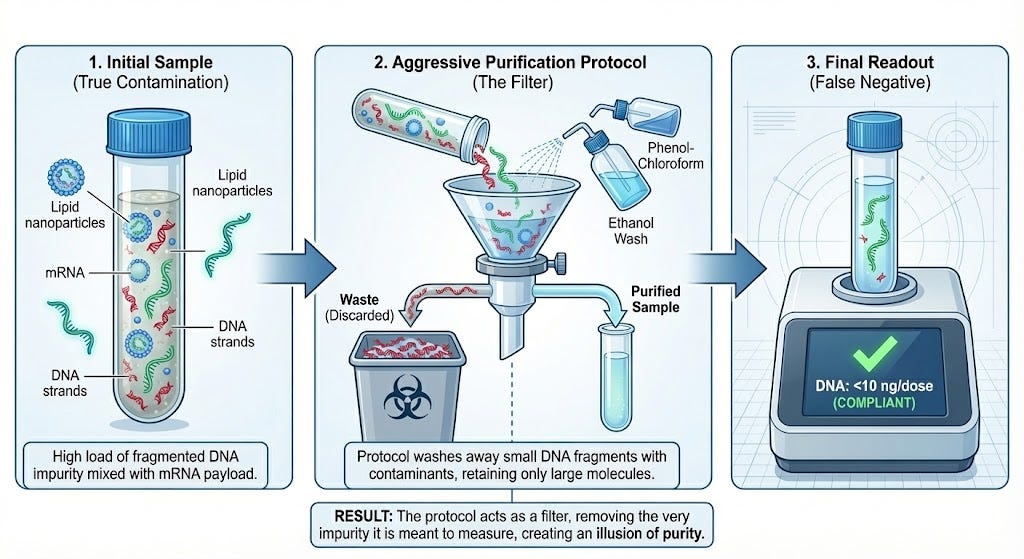
The “Washing Machine” Problem. To measure the DNA, Achs et al. used a purification method that “washes” the sample. Unfortunately, this method is known to wash away small DNA fragments. Since most of the DNA contamination consists of these small fragments, Achs et al. essentially flushed a large proportion of the DNA down the drain and then tested the cleaner portion, declaring it safe. Speicher et al. measured the “dirty” water directly and found the contamination.
The “Cooking” Error. To identify the DNA, Achs et al. heated the vaccine samples to 95°C (203°F) for 10 minutes. This is hot enough to physically break large, intact DNA strands into tiny, harmless-looking pieces. They then reported that the DNA was “naturally” broken and harmless. Speicher et al. did not boil the samples and found large, intact DNA strands (plasmids) that are potentially biologically active.
The “False Alarm” Defense Failed. Achs et al. claimed the high readings in Speicher et al. were just a technical glitch (the machine mistaking RNA for DNA). This was proven wrong. When a chemical that only destroys DNA was added to the sample, the signal vanished. This proves the material was indeed DNA, not a glitch.
Conclusion
The claim that the vaccines are free of excessive DNA relies on testing methods that are blind to the problem. When tested with methods that preserve the sample, the vaccines are found to contain residual DNA levels 4 to 500 times above the safety limit. This DNA is packaged in a way that allows it to enter human cells and potentially function, raising significant safety questions that were missed by standard checks.
Full Report
As a scientist, when two datasets regarding the same pharmaceutical product stand in direct contradiction, the immediate—and correct—impulse is not to defend one’s own work, but to audit the methodologies.
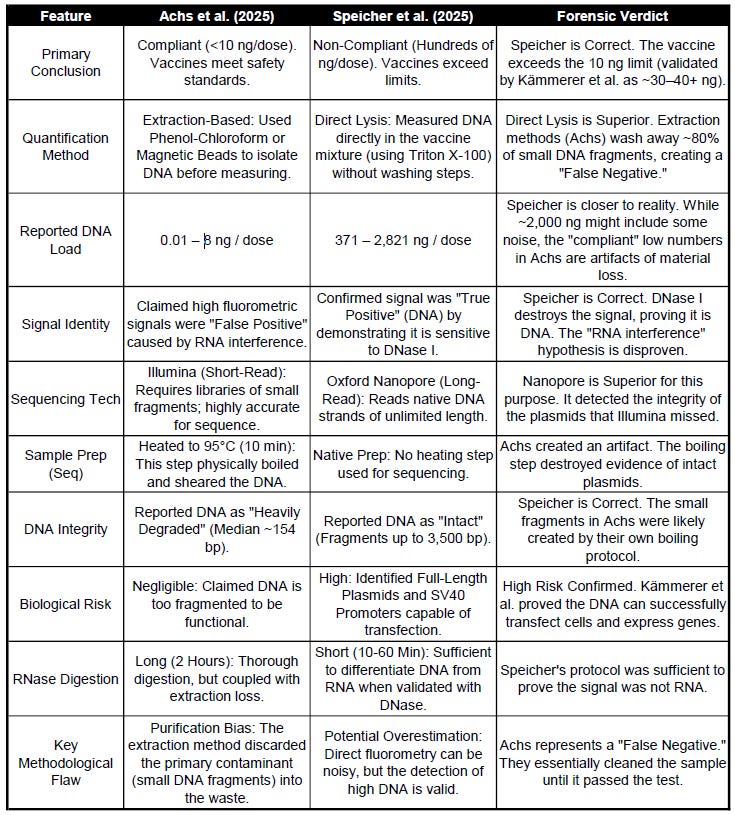
On one hand, our study (Speicher et al) published findings indicating that COVID-19 mRNA vaccine vials contained residual DNA levels exceeding regulatory limits by hundreds of fold1. On the other hand, a recent study by Achs et al. (2025) claims to have definitively proven that these vaccines are compliant, reporting DNA levels consistently below the 10 ng/dose limit2.
On the surface, the Achs et al. manuscript appears to be the methodologically superior study, projecting an image of definitive rigor through its use of four orthogonal analytical approaches—qPCR, fluorometry, capillary electrophoresis, and Next-Generation Sequencing—to cross-validate its findings. The authors frame their work as a systematic corrective to "improperly conducted analyses," specifically critiquing previous reports for failing to rigorously control for RNA interference, which they argue inflated the results in studies like those by Speicher et al.. By ostensibly validating their low fluorometric readings with capillary electrophoresis traces that showed no visible DNA smears , Achs et al. constructed a compelling narrative that their extensive purification protocols yielded the "true" result, portraying the high DNA levels reported by Speicher as mere technical artifacts caused by insufficient RNA digestion.
To determine the ground truth, I undertook a forensic re-evaluation of the methodologies used in both studies, alongside data from König & Kirchner (2024)4 and Kämmerer et al. (2024)3. This analysis was conducted objectively, deriving conclusions solely from the chemical and physical constraints of the protocols employed.
The results of this audit are disturbing. They suggest that the “safe” results reported by Achs et al. are not a reflection of vaccine purity, but an artifact of aggressive purification protocols that systematically removed the contaminant before it could be measured.
1. The Quantification Discrepancy: The “Washing Machine” Effect
The central conflict lies in the Total Mass of DNA. My team utilized a “direct lysis” method, measuring the DNA directly in the vaccine mixture after dissolving the lipid nanoparticles (LNPs). We found values ranging from 371 to 2,821 ng/dose. Achs et al. reported values of <10 ng/dose, arguing that my high values were false positives caused by “RNA interference”—essentially, that the fluorescent dye was detecting the mRNA payload rather than DNA.
To “correct” for this, Achs et al. employed rigorous DNA extraction methods involving Phenol-Chloroform and magnetic beads.
The Forensic Flaw:
DNA extraction is not a neutral process. Standard extraction protocols, particularly those relying on ethanol precipitation (as used by Achs and Kaiser et al.), are known to have poor recovery rates for small DNA fragments.
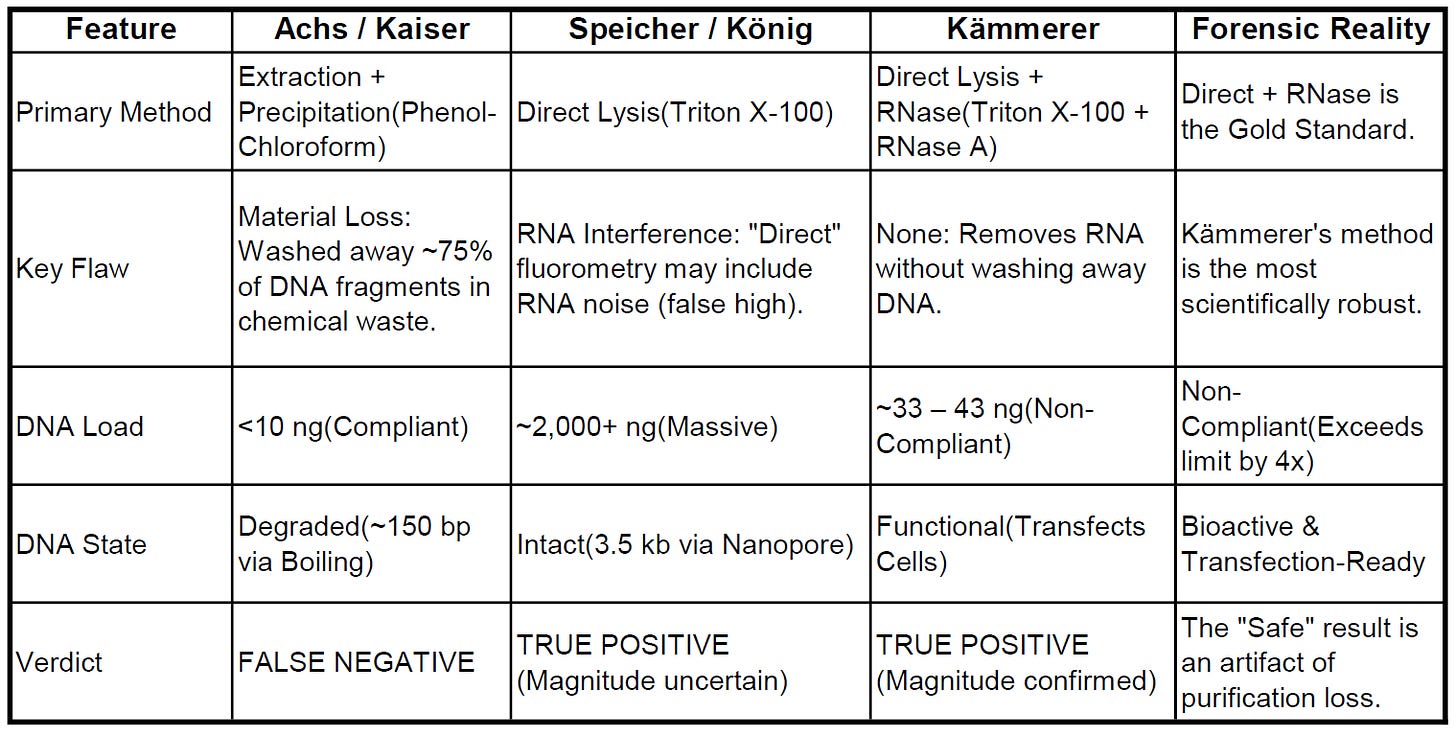
This limitation was empirically exposed by Kämmerer et al. (2024). Unlike Achs, who washed the sample, Kämmerer used a “Direct Lysis + RNase” method—removing the interfering RNA without subjecting the sample to precipitation.
The Result: Kämmerer detected 32.7–43.4 ng of DNA per dose 3.
The Implication: This value is roughly 4 times the regulatory limit. By comparing this to the <10 ng result from extraction-based studies, it becomes mathematically evident that the extraction protocols used by Achs likely washed away ~75% of the DNA contaminant.
My forensic conclusion is that Achs et al. did not measure the total DNA in the vial; they measured only the precipitable DNA. Their protocol acted as a filter, discarding the bulk of the fragmented DNA with the chemical waste.
2. The Characterization Failure: An Artifact of Boiling
A second major discrepancy involves the physical state of the DNA. Achs et al. describe the residual DNA as “heavily degraded” with a median fragment size of ~154 bp. They argue this makes the DNA biologically inert.
In contrast, our study used Oxford Nanopore sequencing and detected intact DNA fragments up to 3,500 bp (3.5 kb). This suggests that full-length plasmids survived the manufacturing process.
The Forensic Flaw:
A review of the Achs et al. methods reveals a critical error in their sequencing library preparation:
“Samples were first heated at 95°C for 10 minutes to release nucleic acids”.
In molecular biology, heating DNA to 95°C for 10 minutes induces thermal hydrolysis, a process that physically shears the phosphodiester backbone of large DNA molecules. The “bell curve” distribution of small fragments (~154 bp) reported by Achs is a textbook signature of thermally degraded DNA.
By boiling the samples, Achs et al. effectively destroyed the evidence of intact plasmids before they could be sequenced. My team avoided this step, which is why we were able to detect the true, larger size of the residual DNA.
3. The “RNA Alibi”: A Hypothesis Disproven by Chemistry
The primary defence mounted by Achs et al. against my findings was that the high Qubit signals were merely “RNA interference.”
The Forensic Test:
To verify the identity of the signal, Kevin McKernan examined the results of a sequential enzymatic digestion:
RNase Treatment: High signals persisted, suggesting the material was not RNA (or was protected).
DNase I Treatment: When treated with DNase I (an enzyme specific to DNA that cannot degrade RNA), the signal vanished to baseline 6.
The Conclusion:
If the signal were RNA, DNase I would have had no effect. The fact that the signal was abolished by DNase I proves definitively that the material was DNA. This chemically disproves the “RNA interference” hypothesis and validates that the high signals observed in my study corresponded to actual DNA mass.
Synthesis of Evidence: The True Status of the Vaccine
By triangulating the data from my own study alongside Achs, König, Kaiser, and Kämmerer, a clear picture emerges. The “Safe” conclusion stands alone as an artifact of material loss and thermal degradation.
Table 1: Synthesis of Evidence
A forensic triangulation of methodology and findings across the major studies.
Table 2: Detailed Comparative Analysis (Achs vs. Speicher)
A head-to-head audit of why the results diverge.
Conclusion: A Failure of Methodology
Based on this rigorous audit, I must conclude that the Achs et al. paper is methodologically unsound for the specific purpose of quantifying fragmented DNA impurities. By relying on purification techniques that discard the target analyte and thermal steps that degrade the sample, Achs et al. engineered a result that appears compliant but does not reflect biological reality.
The independent studies—including my own—provide a consistent signal: the vaccines contain transfection-competent, bioactive DNA at levels that exceed the 10 ng safety limit. The “safety” of these products, as presented by Achs et al., is a methodological illusion.
References
Speicher, D.J., Rose, J., & McKernan, K. (2025). Quantification of residual plasmid DNA and SV40 promoter-enhancer sequences in Pfizer/BioNTech and Moderna modRNA COVID-19 vaccines from Ontario, Canada. Autoimmunity, 58(1), 2551517.
Achs, A., Sedlackova, T., Predajna, L., et al. (2025). Systematic analysis of COVID-19 mRNA vaccines using four orthogonal approaches demonstrates no excessive DNA impurities. npj Vaccines.
Kämmerer, U., Schulz, V., & Steger, K. (2024). BioNTech RNA-Based COVID-19 Injections Contain Large Amounts Of Residual DNA Including An SV40 Promoter/Enhancer Sequence. Science, Public Health Policy, and the Law, 5, 2019-2024.
König, B., & Kirchner, J.O. (2024). Methodological Considerations Regarding the Quantification of DNA Impurities in the COVID-19 mRNA Vaccine Comirnaty®. Methods and Protocols, 7(41).
Kaiser, S., Kaiser, S., Reis, J., & Marschalek, R. (2025). Quantification of objective concentrations of DNA impurities in mRNA vaccines. Vaccine, 55, 127022.
McKernan, K. (2025). Grok Gone Wild. Anandamide (Substack) [https://anandamide.substack.com/p/grok-gone-wild].
Support the Science
I have and will always keep this Substack free, as I don’t agree with censoring information that needs to be shared. However, with Christmas approaching and winter setting in, it has been difficult to continue in the fight and provide for my family.
If you are able and wish to support my research further, there are several options.
Courageous Truth is reader supported, consider being a paid subscriber.
Contact me directly via e-mail: research@davidspeicher.com.
Send an e-transfer to support@davidspeicher.com
Support my Give Send Go campaign.
Follow me on X. @DJSpeicher



I would like to believe the Achs analytical errors were unintended but I highly doubt it. The strategy of designing studies to get an intended result is as old as the hills. Unfortunately this strategy proves to be effective in deceiving the corporate media, public and government due to willful blindness. Keep up the good work, and Merry Christmas to you and your family.