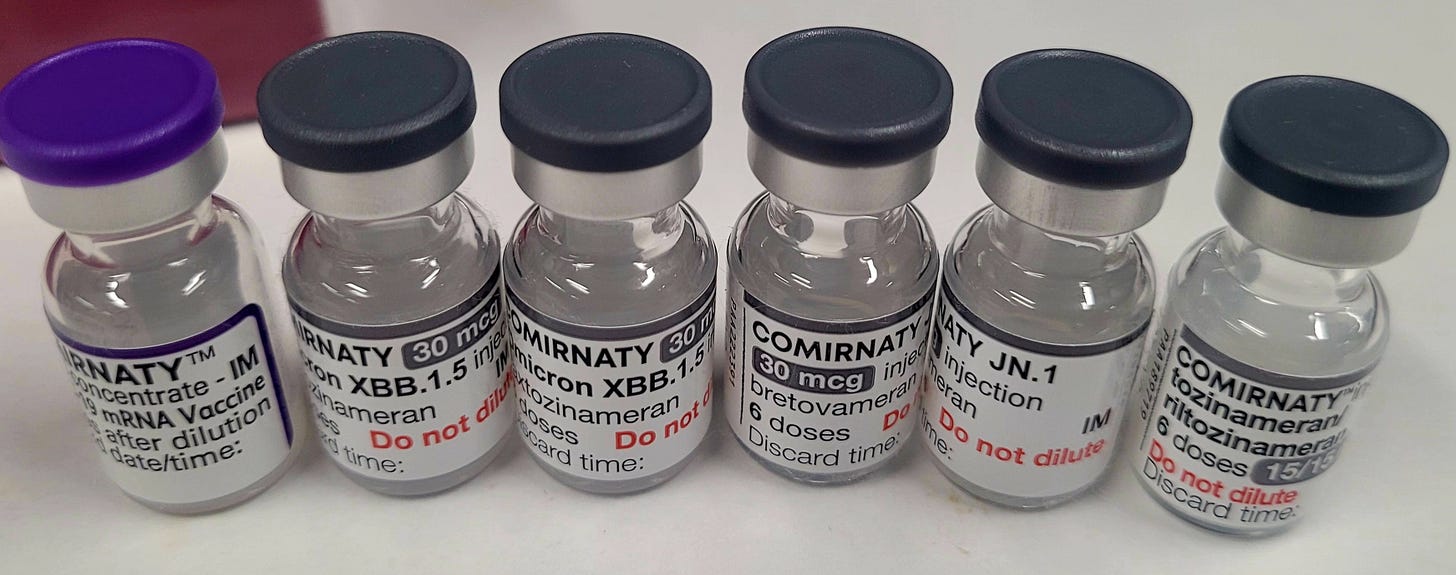
Pfizer Comirnaty JN.1 booster contains high levels of residual plasmid DNA and SV40 promoter-enhancer DNA sequences.
Happy St. Patrick's Day to the brave scientists and doctors and those who heeded their warnings.
To the brave doctors and scientists doing what they can to help those in need…
‘‘May your blessings outnumber the shamrocks that grow, and may your bravery shine brighter than the gold at the end of the rainbow.”
In late 2024, an Irish doctor concerned about the DNA contamination and SV40 promoter-enhancer sequences sent me six Prizer Comirnaty COVID-19 modRNA vials for testing. This included an original purple-topped vial (PBS buffer formulation), two of the XBB.1.5. booster, and two of the brand new JN.1 booster. All vials were unopened and the XBB.1.5 and JN.1 boosters were unexpired.
The questions we were trying to determine were:
Do the new boosters contain just as much residual plasmid DNA as the previous Pfizer COVID-19 modRNA vaccines?
Has the SV40 promoter-enhancer been removed in the new JN.1 booster?
Is this truly a plug-and-play system that Pfizer uses with no intention of removing the DNA contamination and SV40 sequences?
I have had several discussions with close colleagues and many have thought that after 18 months of scrutiny into DNA contamination and the SV40 sequences Pfizer would have cleaned up their vaccines. Now we get to test that hypothesis and see if Pfizer did clean up the vaccine or are the new boosters as contaminated as the previous versions.
Vaccine Vials Tested
The Irish vials tested are shown in the pictures below. The vials came unopened and tamper-free. The XBB.1.5 and JN.1 boosters were in code (i.e. unexpired). Two Canadian vials, a purple-topped (PBS formulation) and grey-topped (Tris formulation), were tested alongside the Irish vials for comparison and as positive controls.
The chain of custody was not documented and cold storage was not kept during shipping. This is not expected to affect residual DNA readings because DNA is stable at room temperature and vials were sealed. Vials were stored in the fridge before and after shipping.
Methods
Due to the reagents available and the fact that this work was not being funded, the vials were only tested for specific DNA sequences by qPCR and not total DNA by Qubit fluorometry. Based on previous work in the Canadian and Australian vials, fluorometry produces much higher DNA yields than qPCR, even after using RNase A to digest the mRNA. When the reagents arrive, these vials will be retested by fluorometry.
The qPCR assay used was from Medicinal Genomics (PathoSEEK® RT-qPCR Master Kit v2; Part# 420207, Beverly, USA) and tested on 2 µL from each vial diluted 1:10 and directly added to 8 µL of master mix.
Cycling was performed on a QuantStudio 3 (ThermoFisher Scientific, Waltham, USA) with an initial denaturation of 95°C for 1 minute followed by 40 cycles of 95°C for 5 seconds and 65°C for 30 seconds.
All qPCR assays used a synthetic gDNA control (gBlock, Integrated DNA Technologies (IDT), San Diego, USA) of known concentration (1 ng/µL) to generate a 10-fold serial dilution derived calibration curve.
As a calibration curve was used QuantStudio software v2.7.0 (ThermoFisher Scientific) produced Cycle of quantitation (Cq) scores ng/µL for each sample. Amplicon mass, as determined with the New England BioLabs DNA calculator, and length (105 bp for ori, 114 bp for spike, 72 bp for SV40 promoter-enhancer-ori) were used to estimate the total nanograms (ng) of DNA present by adjusting for the length of the plasmids (7,824bp for Pfizer and 6,777bp for Moderna). The PCR copy number/dose and the total DNA as determined by fluorometry was adjusted first for the dilutions and then for the volume of each intramuscular vaccine injection dose used clinically (300 µL for Pfizer) to provide a final ng/dose value.
Results
Standard curves of the qPCR assay were nearly perfect with positive and negative controls amplifying as expected (i.e. positive were positive and NTC and negative control not amplifying).
The amplification curve of the Pfizer Comirnaty JN.1 booster was as follows.
The table below gives the cycles (Cq), calculated ng/dose, and total copies/dose for spike, ori (the plasmid’s origin of replication), and the SV40 promoter-enhancer sequence for the Irish vials. IP002 and IP003 are the XBB.1.5 booster and IP004 and IP005 are the JN.1 booster.
The graphical representation shows the extent to which the various targets are over the current 10 ng/dose guideline.
Importance
It cannot be stressed enough that the 10 ng/dose guideline as established by the WHO/FDA and used by most global regulatory agencies, is for “naked” DNA. "Naked DNA" refers to DNA that is not associated with proteins, lipids, or other molecules for protection or packaging, often used as a non-viral vector in gene therapy and DNA vaccination. These guidelines are for naked DNA fragments ≤200 bp and not for protected synthetic DNA inside lipid nanoparticles (LNPs). The guidelines also do not account for multiple dosing of the same vaccine or platform, the risk of regulatory sequences, integration of small DNA fragments (7bp to 200 bp), or nuclear entry/integration.
It is also important to note that over the decades the WHO has allowed the threshold of plasmid DNA per dose to become more lenient and increase from 10 pg/dose to 10 ng/dose. Also, in January 2003 plasmid DNA-based vaccines were given “privileged status” thus allowing far less strict safety protocols. When we put both the historic and current guidance for naked DNA on the logarithmic graph it is clear at how far above the acceptable limits the DNA in the Pfizer COVID-19 vaccines actually are. Given that the DNA is not naked but encapsulated in the lipid nanoparticles, which are 10-100X more efficient at transfecting cells the limit for modRNA vaccines should be somewhere between the two lines. Even at that cut-off level the DNA detected in the Irish vials, including the JN.1 boosters, is way too high.
When compared to the Canadian and Australian vials, the Australian FN0565 contains the highest plasmid DNA load that I have seen, but the Irish vials are slightly higher than the Canadian vials. It is also important to note that the differences in loads (ng/dose) between the targets (i.e. spike, ori and SV40) is due to the differences in digestion with DNase I in the manufacturing process with the ori digested easier than spike or SV40.
A Note on Variants
In September 2024 Health Canada recalled all XBB.1.5 boosters possibly to make room for the new boosters. By that time the XBB lineage had been superseded by the JN lineage for nearly 12 months. Even the current boosters are at minimum 6-12 months out of date. In Europe, it is the JN.1 boosters that are currently being administered whereas in Canada it is the KP.2 boosters.
The JN.1 is a variant of SARS-CoV-2 that emerged as a descendant of the BA.2.86.1 (also known as "Pirola") lineage, with an additional S:L455S substitution in its spike (S) protein. It became dominant by early 2024, out-competing the previous XBB lineage due to its increased fitness and immune evasion capabilities. JN.1 is antigenically distinct from earlier variants like XBB.1.5, which is the target of current monovalent COVID-19 vaccines.
The KP.2 is a sublineage or descendant of JN.1, specifically labelled as JN.1.11.1.2. It evolved from JN.1 and carries additional spike protein mutations compared to its parent variant, making it a more recent and rapidly spreading offshoot.
Conclusion
While the JN.1 booster is being administered in Europe and the KP.2 booster in Canada based on these findings it’s clear that Pfizer COVID-19 modRNA vaccines use a plug-and-play system that does not get clearer with time. The new boosters have equally high levels of residual plasmid DNA and SV40 sequences. This is a “plug-and-play” system as Pfizer only needs to change the spike DNA fragment of the plasmid to change which variant(s) their vaccine targets. This is why there are high levels of SV40 sequences in all models.
While I have not yet tested a KP.2 vial from Canada I presume that it would not be any better than the JN.1 in Europe.
Related Presentations
This date on the Irish vials and current JN.1 booster was presented March 3, 2025 at An Injection of Truth in Calgary. I was the first presenter.
It was also presented in an interview with John Campbell with both a summary video and full interview published.
Support the Science.
My family and I are deeply encouraged and thankful by the number of people who have supported us in recent months…either financially or with encouraging messages. The numerous blessings have helped us in our struggles and we are encouraged to see where 2025 will take us.
If you want to support my research further, here are several options.
Courageous Truth is reader supported, consider being a paid subscriber.
Contact me directly via e-mail: research@davidspeicher.com.
Send an e-transfer to support@davidspeicher.com
Support my Give Send Go campaign.
Follow me on X. @DJSpeicher